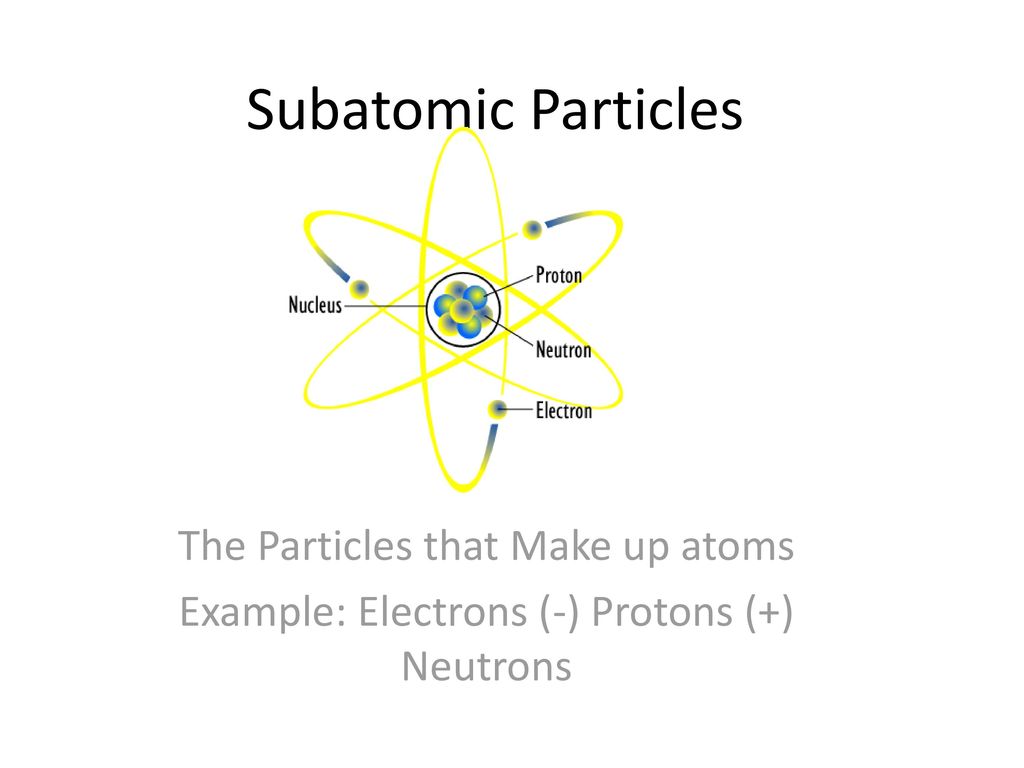
3 Subatomic Particles That Make Up an Atom
Atoms are the fundamental components of matter. Protons neutrons and electrons.

Atomic Structure And Subatomic Particles Youtube
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons-Positive Ion - Occurs when an atom loses an electron negative charge it has more protons than electrons-Negative Ion - Occurs when an atom gains an electron negative charge it will have more electrons than protons.
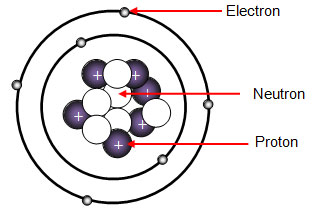
. The number of protons that an atom has is called the atomic number and determines the identity of the atom. What subatomic particles make up an atom. Answer 1 of 3.
There are three subatomic particles. Leave a Reply Cancel reply. Two of the subatomic particles have electrical charges.
At 10-11 seconds the weak nuclear force split form the others allowing formation of the first quarks the building blocks of subatomic particles. Protons neutrons and electrons. Which subatomic particles make up most of the mass of the atom.
The number of protons is always equal to number of. It has no charge. The majority of an atoms mass comes from the protons and neutrons that make up its nucleus.
Electrons are very tiny particle that surround the nucleus and is negatively - charged Thomsons model first scientist to suggest an atom contains smaller. They also help contribute to the atomic weight. Two of the subatomic particles have electrical charges.
Protons including neutrons give the atom mass but are not involved in chemical reactions. The neutron having no charge is neutral. During this explosion the first subatomic particles that make up matter and energy were created.
Protons neutrons and electrons. Subatomic particles include electrons negatively charged nearly massless particles that account for much of the atoms bulk that include the stronger building blocks of the atoms compact yet very dense nucleus the protons that are positively charged and the strong neutrons that are electrically neutral. What are the 3 subatomic particles that make up an atom.
What are the 3 subatomic particles that make up an atom. Which statement must be true for this atom to have no net electrical charge. Terms in this set 3 Which two subatomic particles make up the nucleus of an atom.
Protons neutrons and electrons. What are the 3 subatomic particles that make up an atom. Which part of an atom has the most mass.
The main three subatomic particles are Protons electrons and neutrons. As a result the existence of various types of matter around us is due o the presence of atoms in them. Protons have a positive charge.
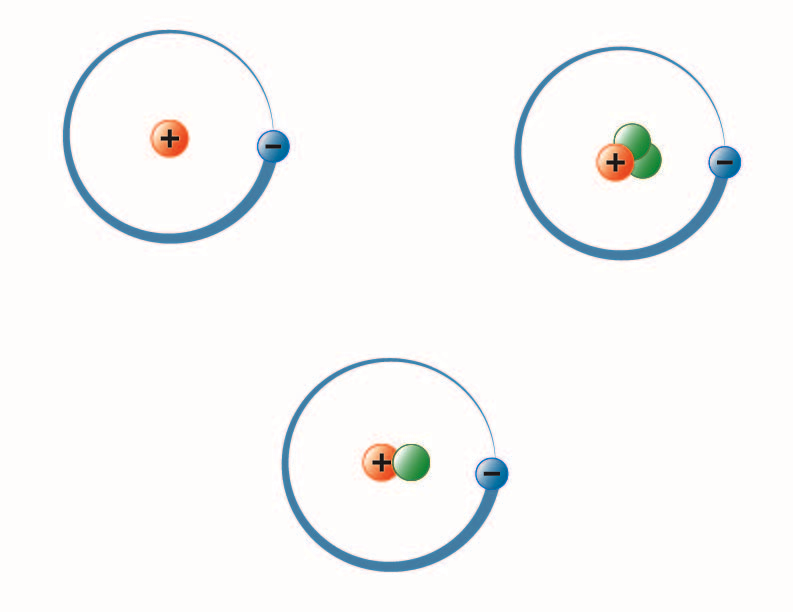
The number of protons also determines the identity of the atom its atomic number. Protons are positively charged and are located in the nucleus. An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no electrical charge.
The three subatomic particles that make up the atom are protons and neutrons inside the nucleus of the atom and electrons which are located in the electron cloud surrounding the atomic nucleus. Given that these particles make up atoms they are often referred to as subatomic particles. What are 3 subatomic particles that make up an atom.
Structure of an atom Nucleus center or core which contains protons and neutrons Proton is a positively charged particle in the nucleus Neutron is neutral. Protons neutrons and electrons. Your email address will not be published.
What subatomic particles make up an atom. Protons neutrons 0 electrons - There are more than three in most everything accept Deuterium 1 of each. Protons neutrons and electrons To.
Protons neutrons and electrons are the three main subatomic particles found in an atom. There are three subatomic particles. Protons have a positive charge while electrons have a negative charge.
The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. An atom itself is made up of three tiny kinds of particles called subatomic particles. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud.
The three common subatomic particles are the proton the neutron and the electron. An atom itself is made up of three tiny kinds of particles called subatomic particles. Required fields are marked Comment Name Email.
Protons electrons and neutrons are the three subatomic particles that typically make up an atom. The atom has 2 negatively charged electrons outside the. Protons neutrons and electrons make up the subatomic particles of an atom.
Helium has 6 and Hydrogen 2 no neutron in its most common form. An atom has 2 protons in its nucleus. Electrons are the least massive of an atoms constituent particles with a mass of 911 x 1031 kg and a size too small to be measured by current techniques.
The protons charge is 1 and is balanced out in a neutral atom by the electrons -1 charge. Protons have a positive charge while electrons have a negative charge.

The Diagram Below Shows The Element Carbon Atoms Are Made Up Of Small Parts Which Are Called Subatomic Particles The Three Main Subatomic Particles That Form An Atom Are Protons Neutrons And Electrons This Activity Might Not Be Viewable On Your

The Chemistry Of Life Objectives What Three Subatomic Particles Make Up Atoms How Are All The Isotopes Of An Element Similar What Are The Two Types Ppt Download
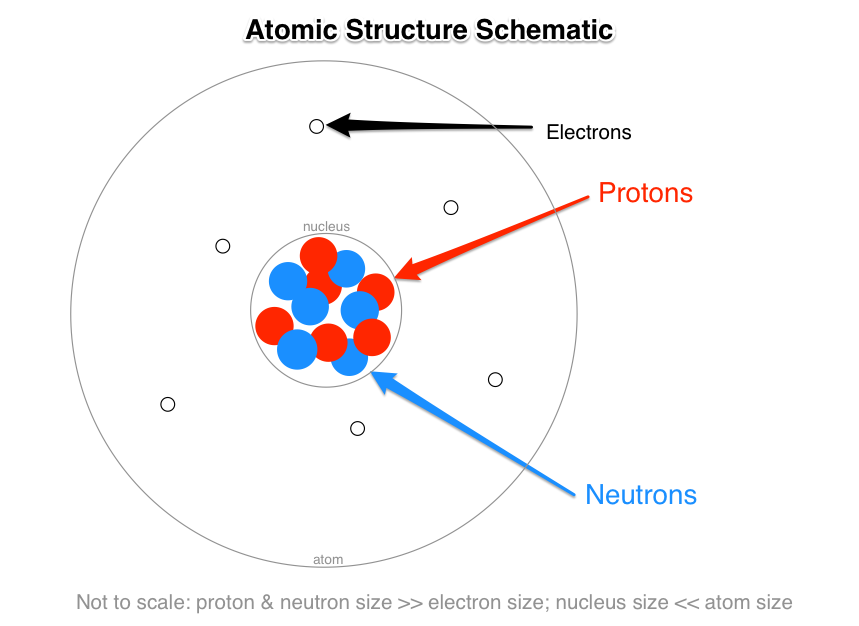
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic
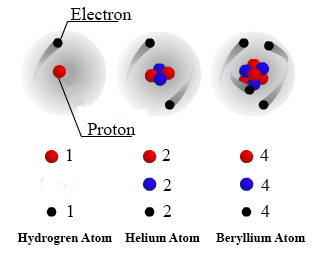
Nondestructive Evaluation Physics Atomic Elements
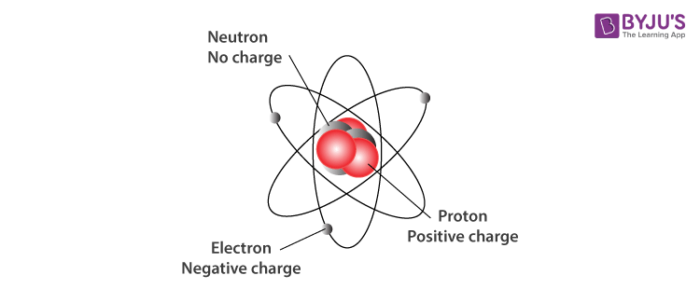
Subatomic Particles Definition Discovery And Key Features
(186).jpg)
Atoms And Elements Test Quiz Proprofs Quiz

Bohr Atomic Model Physics Atomic Theory Atomic Structure Bohr Model
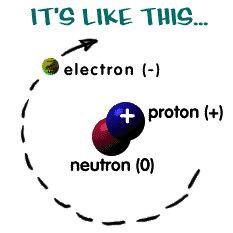
What Are The Three Subatomic Particles Found Inside An Atom Socratic
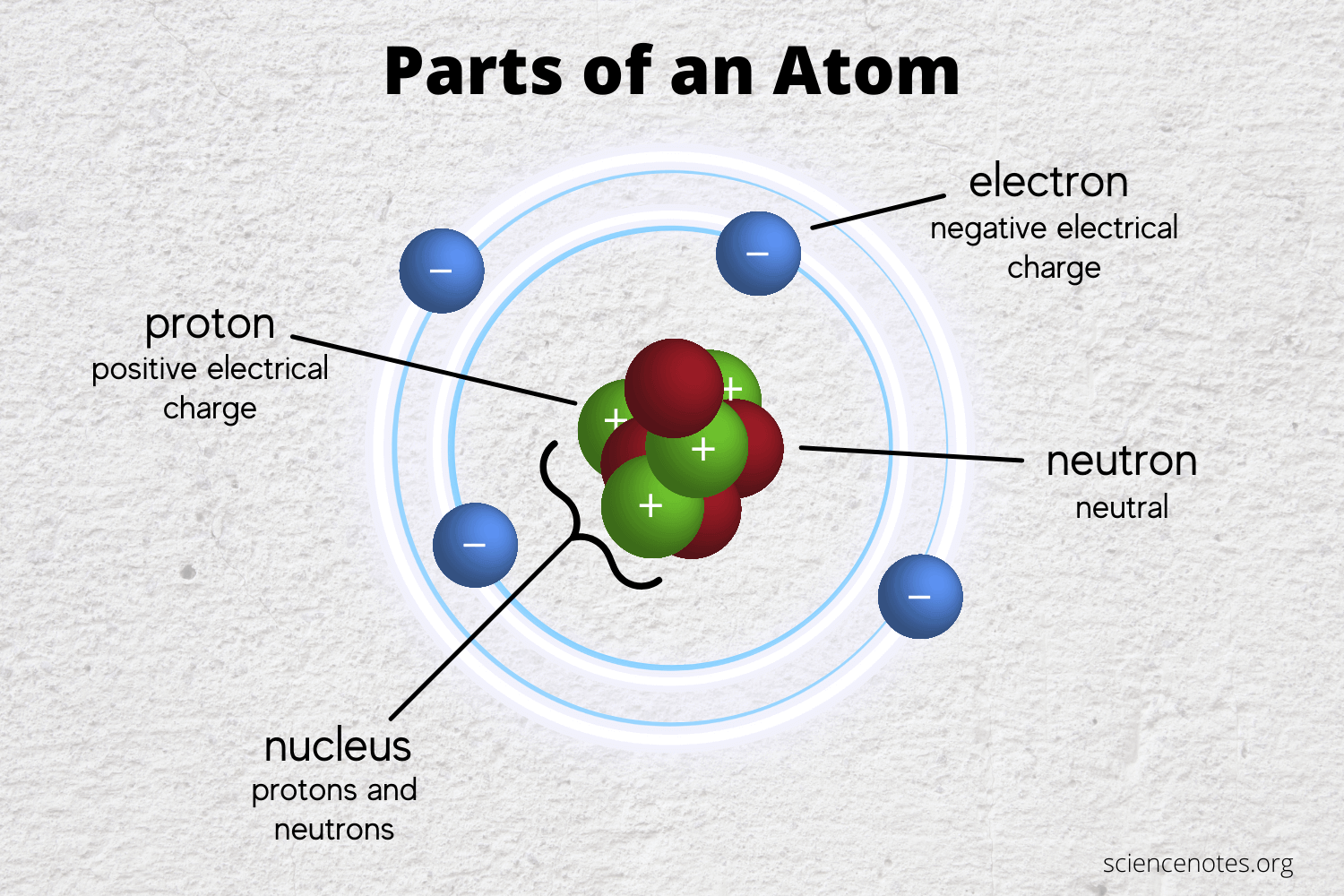
The Famous Types Of Subatomic Particles Praxilabs
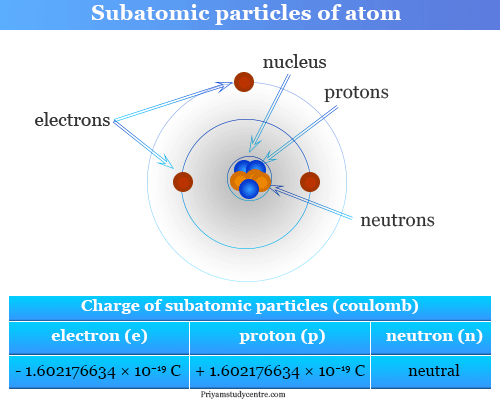
Elementary Particles Subatomic Particles List Mass Charge

Chapter 2 The Chemistry Of Life Objectives What Three Subatomic Particles Make Up Atoms How Are All Of The Isotopes Of An Element Similar Calculate The Ppt Download

Lesson Overview Lesson Overview The Nature Of Matter Lesson Overview 2 1 The Nature Of Matter Ppt Download
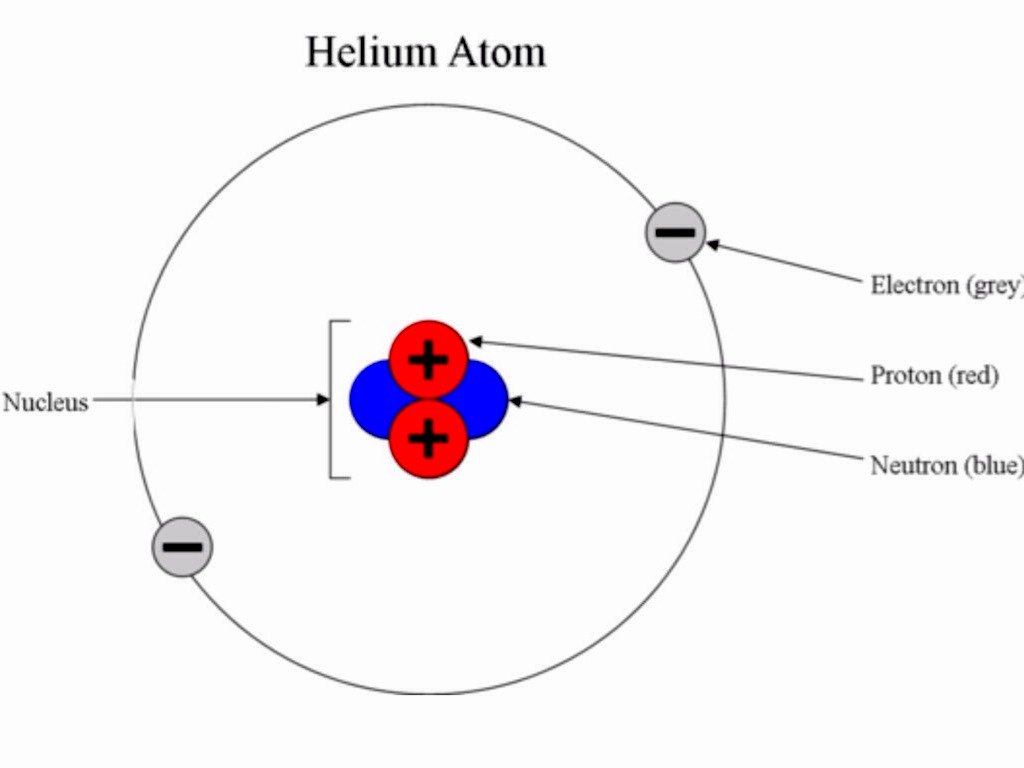
Subatomic Particles By Andrea Cleofas

2 1 Elements And Atoms The Building Blocks Of Matter Anatomy Physiology

The Three Particles That Make Up An Atom Are A Protons Neutrons And Isotopes B Neutrons Isotopes And Electrons C Positives Negatives And Ppt Download

Subatomic Particles Charge Mass What Are Subatomic Particles Video Lesson Transcript Study Com
Subatomic Particles The Particles That Make Up Atoms Ppt Download

Comments
Post a Comment